Used cooking oil polymer combats mercury pollution
Waste cooking oil from cafes and fast food outlets can be used to capture mercury pollution from sites contaminated by agriculture, mining and other industry.


Sulphur + cooking oil = magic mercury polymer.
The technology developed at Flinders University in South Australia is being trialled at mine and farming sites as plans for commercial production take shape.
Mercury leaching into the environment after mining and burning fossil fuel can be a biological disaster, and will soon be regulated by a United Nations convention to prevent harm to humans.
The Flinders University team of chemists led by Dr Justin Chalker developed a polymer called Sulfur-Limonene Polysulfide (SLP) from orange oil in 2015 to tackle the issue.
However, the relatively high cost of SLP, its limited application and lack of durability to handle a serious field trial led the team to look for alternatives.
“A lot of these sites where we want to remediate mercury – gold mines, chlor-alkali plants and some parts of agriculture where they use mercury-rich fungicides – you need a lot of the material and in order for that to be viable it needs to be very, very cheap,” Dr Chalker said.
“So we looked for the most inexpensive plant oils we could find and canola oil – and better yet waste cooking oil – cuts the cost of the raw materials down by orders of magnitude.
“On top of that it’s more durable, it survives higher temperatures and it can capture a much more diverse range of mercury species more like the ones you would encounter in the field.”
The new polymer only has two ingredients; second-hand canola oil and sulphur – a common, low-cost by-product from petroleum production
It can be used in remediation of soil, water and air.
After absorbing mercury pollution the rubber-like polymer changes colour from brown to black to indicate the job is done. The mercury remains permanently bound in the polymer and can be safely stored without further environmental risk.
Published in Chemistry – A European Journal this month, Dr Chalker said the team had shown that the canola oil-based sulphur polymer could capture organic mercury, inorganic mercury, mercury of metal and the gaseous form of mercury.
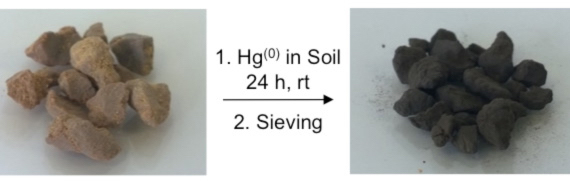
A polymer made from canola oil and sulphur (left) can remove mercury metal from soil. After reacting with mercury metal, the polymer changes colour from brown to black.
“That makes it much more general and much more suitable for the types of problems we are trying to solve in the environment,” he said.
“In addition to mercury capture we are also looking at other forms of pollution and other applications for the material.”
The Minamata Convention on Mercury is a global treaty to protect human health and the environment from the adverse effects of mercury. The treaty, which has been ratified by 73 nations, comes into force next week.
Dr Chalker said the convention provided an additional opportunity for products such as his team’s canola oil-based polymer.
“In any industry where mercury is emitted or used intentionally they will most likely be obligated legally to have a clear plan for controlling those emissions and in cases where there is no technology that they can feasibly use at a reasonable price they’ll be forced to find other alternatives and we’re hoping we can fill that gap,” he said.
The mercury-binding polymer is licensed for sale to Kerafast, an American reagent company whose primary aim is to make unique laboratory-made research tools easily accessible to the global scientific community.
“That will only satisfy a need for establishing commercial partnerships and what we are doing now is working with some engineering firms in Adelaide to design pilot plants and reactors so we can make a tonne or more of this material,” Dr Chalker said.
“We think that will be in the next year and by that stage we will know the scale on which this polymer can be used.
“There are other materials you can buy right now such as activated carbon but we think that our material will be far cheaper, safer and can be produced on as large or a larger scale than these materials and we’re certainly looking to sell it globally.”
The waste cooking oil sourced for the research so far has been sourced from local eateries in the South Australian capital.
“My students have a good laugh when I go down to the local café or fish and chip shop and come back with a bucket of waste oil that we use to make these polymers,” Dr Chalker said.
“The oil would in most cases be sent to centres that convert it into bio-diesel but we think this can be another sector where repurposing that waste into something that benefits the environment is a good thing.
“It also means that the raw materials for our polymer are going to be cheap and that’s the main criteria that we are trying to satisfy.”
Dr Chalker led the research with several researchers from the Flinders Centre for NanoScale Science and Technology. Key contributions were made by senior collaborators at the University of Cambridge and the Institute for Molecular Medicine in Lisbon in Europe, the Oak Ridge National Laboratory in the United States, and Royal Melbourne Institute of Technology and the University of Melbourne in Australia.





