Why do we allow the health system to fail women?
Despite numerous scandals affecting Australian women, why are substandard medical products still being allowed in the market?
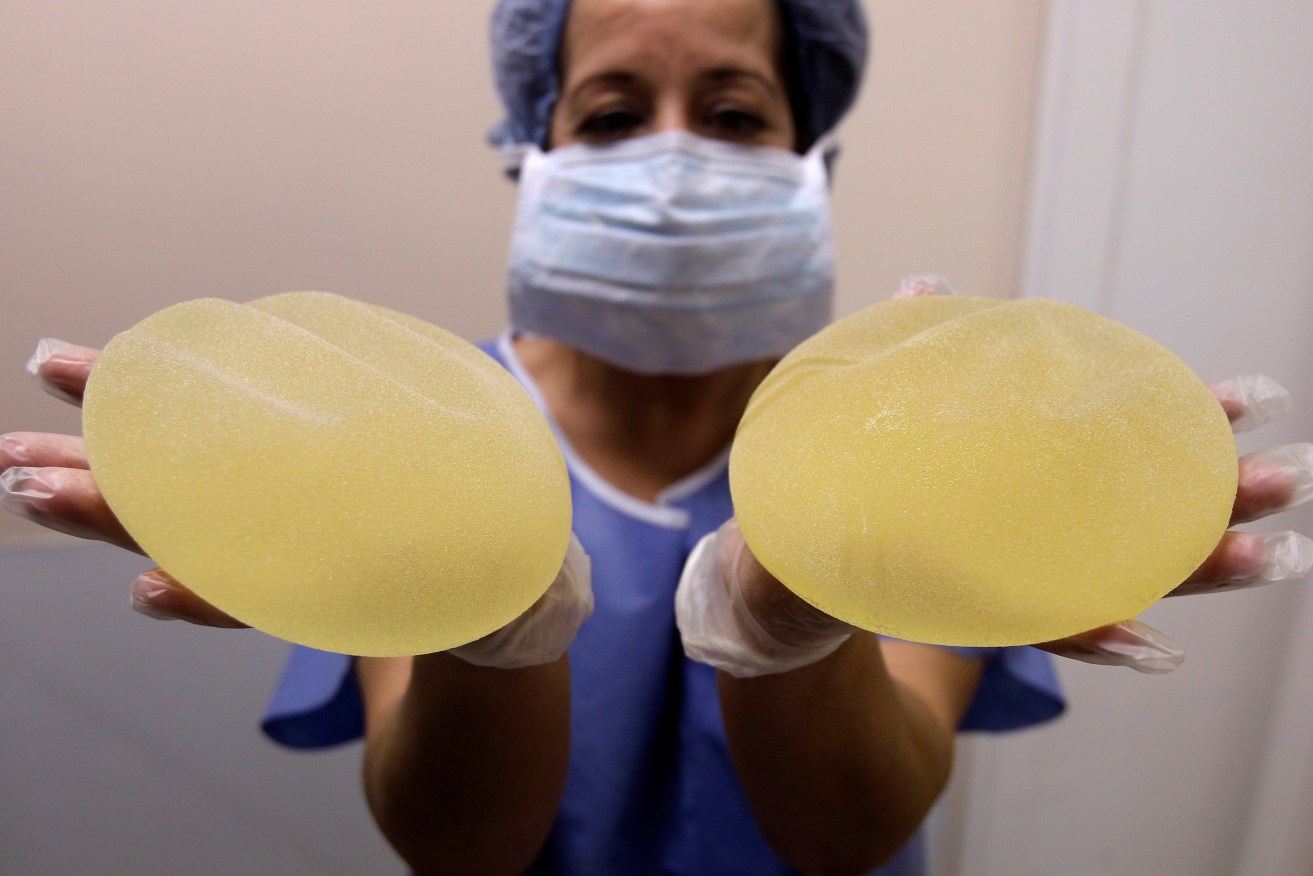
A nurse holds defective breast implants manufactured by French company Poly Implant Prothese (PIP). Photo: AP/Lionel Cironneau
Breast implants, implantable contraceptive devices, vaginal mesh used in pelvic floor repair surgeries and bargain-buy cosmetic procedures… These are meant to improve the health and self-esteem of Australian women. However, a poorly-regulated industry with little financial or legal consequence for questionable operators means thousands of women’s lives are being destroyed by health problems resulting from faulty medical devices or poorly-skilled surgeons.
When we think about undergoing surgery, there are many factors we take into account. How risky is the surgery? How long will I need off from work? How much will it cost? Who will look after my children while I recover?
However, there are further important questions we rarely think to ask:
- How experienced is the surgeon?
- What device will the surgeon be using?
- What is it made out of? Is it safe?
- How do I find out what device is inside me if things go wrong?
The truth of the matter is we place so much trust in doctors that we often don’t ask whether the device being used is safe or whether it has been thoroughly tested. Furthermore, we expect that a record is being kept of the brand and serial number of any medical device that is used in case of emergency such as a product recall – much in the same way global car manufacturers can recall faulty parts that may put lives at risk.
Incredibly, and despite multiple cases of medical devices failing and women becoming sick, a compulsory register is still not in place to record the devices being used in each surgery. When these devices fail, women are forced to deal with the consequences alone, which can involve numerous additional corrective surgeries and treatments, significant medical bills and long-lasting pain and suffering.
After assisting thousands of women who have been devastated by substandard medical devices, I can’t express how important it is for any woman considering surgery to ask these questions and get the medical and legal assurances they need.
Australia has received enough warning signs. In 2012, the PIP breast implant scandal was exposed – a case in which I was heavily involved, both in Australia and internationally. The implants were supplied to Australian women for more than 10 years before it was revealed that they contained industrial-grade silicone, not medical grade, and were prone to rupture, leaking the contents into the body.
The measures needed to protect women from further illness, even death, are clear and easy to implement.
The women who received the PIP implants suffered from a variety of serious side effects including severe pain in the chest, arm and back, silicone storing in the lymph nodes and lymphatic system and resulting arm pain, irregular breast shape, the need for multiple further surgeries, as well as other autoimmune type symptoms.
At the time the fault was discovered many did not know, and had no way of finding out, whether they had received PIP breast implants or not. This was because there was no breast implant registry. Women were commonly not given details of the implants at the time of surgery and by the time the defect became known their surgeon may have either retired or destroyed their medical notes.
Many of these women received PIP breast implants following a mastectomy procedure post-breast cancer, or to remedy asymmetrical breasts. The last thing they wanted was to undergo further surgery just to find out what kind of implants they had. Sadly, for many, that was their only option.
A federal Senate inquiry followed the exposure of the PIP scandal. It published a long list of recommendations including the establishment of “an opt-out Breast Implant Registry as a priority”. Despite the priority status this recommendation was given, a compulsory registry – essential in a time of crisis – has still not been established. This means that if another high risk medical device, such as a breast implant, is recalled it will be impossible to know who and how many women have received the implant in Australia. This is unacceptable. However, one would have thought the need for such a resigtry was obvious, noting that, in an orthopaedic setting, there has been a compulsory register for knee and hip implantable devices since 1999.
A compulsory registry is only now being discussed due to the recent research which found a blood cancer risk associated with breast implant surgery. However, the establishment of a registry has been discussed before, to no avail.
Next, we heard of the substandard Johnson and Johnson vaginal mesh implants. Women received this device, via surgical insertion, to repair pelvic floor damage caused by pregnancy, child birth and menopause, and results in embarrassing symptoms such as urinary incontinence. The complications experienced by the thousands of Australian women who received this implant included the mesh or tape eroding into surrounding tissues and organs, infections, incontinence and chronic pain.
“You get what you pay for”
Cosmetic surgery today is big business and the barrier to entry has never been lower for those wanting to work in the field. Recently, the ABC’s Four Corners exposed the race to the bottom that is Australia’s cosmetic surgery industry, where doctors who aren’t trained plastic surgeons can perform a wide range of invasive surgical procedures.
A plastic surgeon is a qualified doctor who has undertaken a minimum of five to seven years of additional training, after their medical degree, to perform cosmetic and reconstructive surgeries. On the other hand, a cosmetic surgeon is a doctor who has completed a medical degree but who has not usually undertaken further specific surgical training. Ironically, cosmetic surgery is not even a formally recognised specialty in Australia.
The potential for complications arising from surgery is clear. If the price point seems too good to be true, it is possibly due to the limited qualifications of the doctor performing your surgery. Australian women need to ask themselves if they are willing to pay the price tomorrow for cheap surgery today.
In many instances, Australians are able to travel to parts of Asia, undergo a cosmetic procedure such as a breast enlargement or tummy tuck, spend a week in a luxurious resort by a pool while they recover and come home for less than the cost of the surgery alone here in Australia.
However, while this two-in-one, holiday and cosmetic uplift, sounds great on paper, as always the fine print reveals the serious shortfalls. In this context, the motto ‘you get what you pay for’ could not be more accurate.
Whether at home or abroad, you need to know the qualifications of the surgeon performing your procedure. In addition, when considering surgery overseas you also need to consider the health and safety regulatory framework of the country you are planning to visit for surgery. Australia is known for its high standards, however this is not guaranteed when you travel overseas for treatment and you may be putting yourself at an increased risk of infection and illness.
Issues with female contraceptives continue to mount. In 2014, I was involved in the investigation into the Yaz and Yasmin oral contraceptive pills which reportedly had a higher risk of blood clots and resulted in serious complications for thousands of women.
Furthermore, the Assure contraceptive device, which involves the insertion of metal coils into the fallopian tubes to prevent pregnancy, has recently been found to cause serious side effects. The Therapeutic Goods Administration reported the device causes “changes in menstrual bleeding, unintended pregnancy, chronic pain, perforation and migration of the device, allergy/hypersensitivity, or immune-type reactions. Some of these reports were considered serious and resulted in removal of the device, which involved abdominal surgery”.
Questions that can’t be answered
To state the obvious, the one thing these medical devices all have in common is they impact women. So we must ask how are these substandard products making it into our market?
Why are these devices not being thoroughly evaluated before being made available?
What is being done to protect female consumers who have received these devices?
What is being done to reinstate female confidence in the medical device regulatory system?
What compensation is available for these women who receive failed medical devices through no fault of their own?
Who pays for the revision surgery? Who pays for the ongoing medical treatment they now require?
Who covers their income when they are unable to return to work?
Unfortunately, we can’t answer these questions. However, from experience I can say that the women affected by the PIP implants received no subsidy from the government for the further surgery required, no obligation was placed on the surgeon to replace the implants, and there was certainly no avenue in Australia for the women to be compensated for the thousands of dollars they were out of pocket.
Why? Because, under Australian law, a supplier of a high-risk medical devices is not required to have product liability insurance. A breast implant is deemed a high-risk medical device. This leaves trusting women exposed and with no choice but to file their case in France – where the implants were manufactured – in an attempt to recoup some of their losses in a country which seems to have fairer laws in place to protect their female consumers.
Enough is enough. Too many women have suffered from the supply of medical devices that are clearly not suitable and have the potential to be life-threatening. We have seen the benefits of a compulsory implantable device registry for orthopaedics and, logically, this should be required for all implantable devices without any further delay.
We need to ask more of our regulatory bodies to make sure devices undergo more rigorous testing. Furthermore, it should be compulsory for the suppliers of these high-risk medical devices to have insurance so if things do go wrong, innocent women are not left high and dry.
The measures needed to protect women from further illness, even death, are clear and easy to implement. To not do so quickly is nothing short of negligent and heartless. The time to act is now.
Olla Kutieleh is a senior associate at Tindall Gask Bentley Lawyers, working in the field of personal injury and medical negligence. She has acted for many victims of the PIP breast implants and women whose breast cancer was misdiagnosed by Breast Screen SA.




